Abstract
Arsenic (As), the toxic metalloid, is taken up by plant roots and transported to different parts of the plant through transporters of the essential elements due to the structural analogy. The analogy of arsenate (AsV) with phosphate enables As (V) to enter plant through phosphate transporter, while, arsenite (AsIII) which is analogous to silicic acid, is taken up by plants through aquaporins. After the uptake, the different forms of As are translocated to shoot via xylem, imposing toxicity to plants that affect their growth and yield, however this depends on the effective concentration of free As anion at particular cellular organelle /site. To this end, the role of transporters becomes crucial as the central and prime regulator of As movement throughout the plant and in various cellular compartments. It is essential to understand the precise roles of different transporters involved in As uptake and transportation to avoid As accumulation and stress in plant. Therefore, this review discusses the transporters namely, phosphate transporters, nodulin 26-like intrinsic proteins, plasma membrane intrinsic proteins, tonoplast intrinsic proteins, C-type ATP binding cassette transporters, arsenical resistance 3 transporter, inositol transporters, multidrug and toxic compound extrusion transporters, and natural resistance-associated macrophage protein transporters, which are involved in As uptake, sequestration, translocation and efflux in plants, with an emphasis on As stress tolerance through the regulation of expression of the different transporters.


Similar content being viewed by others
Abbreviations
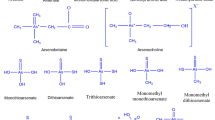
- As:
-
Arsenic
- As(III):
-
Arseite
- As(V):
-
Arsenate
- MMA:
-
Monomethylarsonic acid
- DMA:
-
Dimethylarsinic acid
- MIPs:
-
Major intrinsic proteins
- ABCC:
-
C-type ATP binding cassette transporters
- Acr3:
-
Arsenical resistance 3 transporter
- INTs:
-
Inositol transporters
- MATE:
-
Multidrug and toxic compound extrusion transporters
- NRT1/PTR:
-
Nitrate transporter 1/peptide transporter
- NRAMP:
-
Natural resistance-associated macrophage protein
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Hussain M (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15(1):59
Ahmad P, Alyemeni MN, Al-Huqail AA, Alqahtani MA, Wijaya L, Ashraf M, Kaya C, Bajguz A (2020) Zinc oxide anoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of As and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 9:825
Ashraf MA, Umetsu K, Ponomarenko O, Saito M, Aslam M, Antipova O, Rahman A (2019) PIN FORMED 2 facilitates the transport of arsenite in Arabidopsis thaliana. BioRxiv. https://doi.org/10.1101/710160
Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamas MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As (OH) 3 and Sb (OH) 3 across membranes. BMC Biol 6(1):1–15
Cao Y, Sun D, Chen JX, Mei H, Ai H, Xu G, Chen Y, Ma LQ (2018) Phosphate transporter PvPht1; 2 enhances phosphorus accumulation and plant growth without impacting arsenic uptake in plants. Environ Sci Technol 52(7):3975–3981
Carey AM, Norton GJ, Deacon C, Scheckel KG, Lombi E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA (2011) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192(1):87–98
Catarecha P, Segura MD, Franco-Zorrilla JM, Garcia-Ponce B, Lanza M, Solano R, PazAres J, Leyva A (2007) A mutant of the arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell 19:1123–1133
Chauhan R, Awasthi S, Indoliya Y, Chauhan AS, Mishra S, Agrawal L, Srivastava S, Dwivedi S, Singh PC, Mallick S, Chauhan PS (2020) Transcriptome and proteome analyses reveal selenium mediated amelioration of arsenic toxicity in rice (Oryza sativa L.). J Hazard Mater 390:122122
Chen Y, Xu W, Shen H, Yan H, Xu W, He Z, Ma M (2013) Engineering arsenic tolerance and hyperaccumulation in plants for phytoremediation by a PvACR3 transgenic approach. Environ Sci Technol 47(16):9355–9362
Chen Y, Sun SK, Tang Z, Liu G, Moore KL, Maathuis FJ, Zhao FJ (2017) The Nodulin 26-like intrinsic membrane protein OsNIP3; 2 is involved in arsenite uptake by lateral roots in rice. J Exp Bot 68(11):3007–3016
Chen JX, Cao Y, Yan X, Chen Y, Ma LQ (2021) Novel PvACR3; 2 and PvACR3; 3 genes from arsenic-hyperaccumulator Pteris vittata and their roles in manipulating plant arsenic accumulation. J Hazard Mater. 415:125647
Chen H, Liang X, Gong X, Reinfelder JR, Chen H, Sun C, Liu X, Zhang S, Li F, Liu C, Zhao J (2021b) Comparative physiological and transcriptomic analyses illuminate common mechanisms by which silicon alleviates cadmium and arsenic toxicity in rice seedlings. J Environ Sci 109:88–101
Danielson JA, Johanson U (2008) Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. BMC Plant Biol 8(1):1–15
Das N, Bhattacharya S, Bhattacharyya S, Maiti MK (2018) Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol Biol 98(1):101–120
Debnath S, Bhattacharyya S, Sarkar S, Chatterjee M (2016) Expression of multidrug and toxic compound extrusion (MATE) genes in response to the presence of arsenic in irrigation water and soil in Rice (Oryza sativa L). Int J Bio-Resour Stress Manag 7(1):88–91
Deng F, Yamaji N, Ma JF, Lee SK, Jeon JS, Martinoia E, Lee Y, Song WY (2018) Engineering rice with lower grain arsenic. Plant Biotechnol J 16:1691–1699
Di X, Zheng F, Norton GJ, Beesley L, Zhang Z, Lin H, Zhi S, Liu X, Ding Y (2021) Physiological responses and transcriptome analyses of upland rice following exposure to arsenite and arsenate. Environ Exp Bot 183:104366
DiTusa SF, Fontenot EB, Wallace RW, Silvers MA, Steele TN, Elnagar AH, Smith AP (2016) A member of the Phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol 209(2):762–772
Dos Santos AL, Chaves-Silva S, Yang L, Maia LGS, Chalfun-Junior A, Sinharoy S, ZhaoBenedito JVA (2017) Global analysis of the MATE gene family of metabolite transporters in tomato. BMC Plant Biol 17(1):1–13
Duan G, Kamiya T, Ishikawa S, Arao T, Fujiwara T (2012) Expressing ScACR3 in rice enhanced arsenite efflux and reduced arsenic accumulation in rice grains. Plant Cell Physiol 53:154–163
Duan GL, Hu Y, Schneider S, McDermott J, Chen J, Sauer N, Rosen BP, Daus B, Liu Z, Zhu YG (2015) Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nat Plants 2(1):1–6
Dwivedi S, Kumar A, Mishra S, Sharma P, Sinam G, Bahadur L, Goyal V, Jain N, Tripathi RD (2020) Orthosilicic acid (OSA) reduced grain arsenic accumulation and enhanced yield by modulating the level of trace element, antioxidants, and thiols in rice. Environ Sci Pollut Res 27:24025–24038
Feng H, Li X, Sun D, Chen Y, Xu G, Cao Y, Ma LQ (2021) Expressing phosphate transporter PvPht2; 1 enhances P transport to the chloroplasts and increases Arsenic tolerance in Arabidopsis thaliana. Environ Sci Technol 55:2276–2284
Fontenot EB, DiTusa SF, Kato N, Olivier DM, Dale R, Lin WY, Chiou TJ, Macnaughtan MA, Smith AP (2015) Increased phosphate transport of Arabidopsis thaliana Pht1;1 by site-directed mutagenesis of tyrosine 312 may be attributed to the disruption of homomeric interactions. Plant Cell Environ. https://doi.org/10.1111/pce.12522
Fu SF, Chen PY, Nguyen QTT, Huang LY, Zeng GR, Huang TL, Lin CY, Huang HJ (2014) Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis. BMC Plant Biol 14:1–16
Gautam A, Kumar N, Dubey AK, Ranjan R, Sahu N, Behera SK, Mallick S (2020) Sucrose plays key role in amelioration of arsenic induced phytotoxicity through modulating phosphate and silicon transporters, physiological and biochemical responses in C3 (Oryza sativa L.) and C4 (Zea mays L.). Environ Exp Bot 171:103930
Ghorbani A, Tafteh M, Roudbari N, Pishkar L, Zhang W, Wu C (2021) Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol Environ Saf 209:111793
Gupta AB, Sankararamakrishnan R (2009) Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. BMC Plant Biol 9(1):1–28
He Z, Yan H, Chen Y, Shen H, Xu W, Zhang H, Shi L, Zhu Y‐G, Ma M (2016) An aquaporin Pv TIP 4; 1 from Pteris vittata may mediate arsenite uptake. New Phytol 209(2):746–761
Herath I, Vithanage M, Bundschuh J, Maity JP, Bhattacharya P (2016) Natural arsenic in global groundwaters: distribution and geochemical triggers for mobilization. Curr Pollut Rep 2(1):68–89
Huang TL, Nguyen QTT, Fu SF, Lin CY, Chen YC, Huang HJ (2012) Transcriptomic changes and signaling pathways induced by arsenic stress in rice roots. Plant Mol Biol 80:587–608
Huang Y, Chen H, Reinfelder JR, Liang X, Sun C, Liu C, Li F, Yi J (2019) A transcriptomic (RNA-seq) analysis of genes responsive to both cadmium and arsenic stress in rice root. Sci Total Environ 666:445–460
Huang Y, He G, Tian W, Li D, Meng L, Wu D, He T (2021) Genome-wide identification of MATE gene family in potato (Solanum tuberosum L.) and expression analysis in heavy metal stress. Front Genet. https://doi.org/10.3389/fgene.2021.650500
Indriolo E, Na G, Ellis D, Salt DE, Banks JA (2010) A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 22(6):2045–2057
Isayenkov SV, Maathuis FJ (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7; 1 is a pathway for arsenite uptake. Febs Lett 582(11):1625–1628
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126(4):1358–1369
Juliao MHM (2020) Genomic identification of MATE, ABC, and MFS transporters in Citrus sinensis and expression analysis of Citrus species interacting with Xanthomonas citri subsp. Citri [Master’s, thesis, Universidade Estadual Paulista (Unesp). http://hdl.handle.net/11449/192658
Kamiya T, Fujiwara T (2009) Arabidopsis NIP1; 1 transports antimonite and determines antimonite sensitivity. Plant Cell Physiol 50(11):1977–1981
Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, Fujiwara T (2009) NIP1; 1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem 284(4):2114–2120
Kamiya T, Islam R, Duan G, Uraguchi S, Fujiwara T (2013) Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1.
Kapilan R, Vaziri M, Zwiazek JJ (2018) Regulation of aquaporins in plants under stress. Biol Res 51(1):1–11
Katsuhara M, Sasano S, Horie T, Matsumoto T, Rhee J, Shibasaka M (2014) Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotech. 31:213–219. https://doi.org/10.5511/plantbiotechnology.14.0421a
Khan E, Gupta M (2018) Arsenic–silicon priming of rice (Oryza sativa L.) seeds influence mineral nutrient uptake and biochemical responses through modulation of Lsi-1, Lsi-2, Lsi-6 and nutrient transporter genes. Sci Rep 8(1):1–16
Kim D, Bahmani R, Modareszadeh M, Hwang S (2020) Mechanism for higher tolerance to and lower accumulation of arsenite in NtCyc07-overexpressing tobacco. Plants 9(11):1480
Klein M, Burla B, Martinoia E (2006) The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. EBS Lett 580:1112–1122
Kumari P, Gupta A, Yadav S (2021) Thioredoxins as molecular players in plants, pests, and pathogens. In: Singh IK, Singh A (eds) Plant-pest interactions: from molecular mechanisms to chemical ecology. Springer, Singapore, pp 107–125
LeBlanc MS, McKinney EC, Meagher RB, Smith AP (2013) Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol 163:1–9
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150(4):2071–2080
Liu J, Li Y, Wang W, Gai J, Li Y (2016) Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genomics 17:223
Luan M, Liu J, Liu Y, Han X, Sun G, Lan W, Luan S (2018) Vacuolar phosphate transporter 1 (VPT1) affects arsenate tolerance by regulating phosphate homeostasis in Arabidopsis. Plant Cell Physiol 59(7):1345–1352
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105(29):9931–9935
Maciaszczyk-Dziubinska E, Wawrzycka D, Wysocki R (2012) Arsenic and antimony transporters in eukaryotes. Int J Mol Sci 13(3):3527–3548
Maciaszczyk-Dziubinska E, Migocka M, Wawrzycka D, Markowska K, Wysocki R (2014) Multiple cysteine residues are necessary for sorting and transport activity of the arsenite permease Acr3p from Saccharomyces cerevisiae. Biochim Biophys Acta BBA Biomembr 1838(3):747–755
Mani A, Sankaranarayanan K (2018) In silico analysis of natural resistance-associated macrophage protein (Nramp) family of transporters in rice. Protein J 37(3):237–247
Modareszadeh M, Bahmani R, Kim D, Hwang S (2021) Decreases in arsenic accumulation by the plasma membrane intrinsic protein PIP2; 2 in Arabidopsis and yeast. Environ Pollut 275:116646
Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C, Dhankher OP (2012) Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res 21(6):1265–1277
Mousavi SR, Niknejad Y, Fallah H, Tari DB (2020) Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep 39:1041–1060
Nussaume L, Kanno S, Javot H, Marin E, Nakanishi TM, Thibaud MC (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci 2:83
Pandey AK, Gedda MR, Verma AK (2020) Effect of arsenic stress on expression pattern of a rice specific miR156j at various developmental stages and their allied co-expression target networks. Front Plant Sci 11:752
Peng F, Wang C, Cheng Y, Kang H, Fan X, Sha L, Wang Y (2018) Cloning and characterization of TpNRAMP3, a metal transporter from polish wheat (Triticum polonicum L). Front Plant Sci 9:1354
Pommerrenig B, Diehn TA, Bernhardt N, Bienert MD, Mitani-Ueno N, Fuge J, Bieber A, Spitzer C, Bräutigam A, Ma V, Chaumont V, Bienert GP (2020) Functional evolution of nodulin 26-like intrinsic proteins: from bacterial arsenic detoxification to plant nutrient transport. New Phytol 225(3):1383–1396
Raab A, Williams PN, Meharg A, Feldmann J (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4(3):197–203
Raichaudhuri A (2016) Arabidopsis thaliana MRP1 (AtABCC1) nucleotide binding domain contributes to arsenic stress tolerance with serine triad phosphorylation. Plant Physiol Biochem 108:109–120
Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Biol 10(3):218–227
Remy E, Cabrito TR, Batista RA, Teixeira MC, Sa-Correia I, Duque P (2012) The Pht1;9 and Pht1;8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol 195:356–371
Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46(9):1568–1577
Sarwar T, Khan S, Yu X, Amin S, Khan MA, Sarwar A, Nazneen S (2021) Analysis of Arsenic concentration and its speciation in rice of different markets of Pakistan and its associated health risk. Environ Technol Innov 2:101252
Schneider S, Schneidereit A, Konrad KR, Hajirezaei MR, Gramann M, Hedrich R, Sauer N (2006) Arabidopsis thaliana INOSITOL TRANSPORTER 4 mediates high affinity H+-transport of myo-inositol across the plasma membrane. Plant Physiol 141:565–577
Schneider S, Schneidereit A, Udvardi P, Hammes U, Gramann M, Dietrich P, Sauer N (2007) Arabidopsis thaliana INOSITOL TRANSPORTER2 mediates high affinity H+-symport of different inositols across the plasma membrane. Plant Physiol 145:1395–1407
Schneider S, Beyhl D, Hedrich R, Sauer N (2008) Functional and physiological characterization of Arabidopsis INOSITOL TRANSPORTER1, a novel tonoplast-localized transporter for myo-inositol. Plant Cell 20(4):1073–1087
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642
Singh M, Singh VP, Dubey G, Prasad SM (2015) Exogenous proline application ameliorates toxic effects of arsenate in Solanum melongena L. seedlings. Ecotoxicol Environl Saf 117:164–173
Singh PK, Indoliya Y, Chauhan AS, Singh SP, Singh AP, Dwivedi S, Chakrabarty D (2017) Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci Rep 7(1):1–13
Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee Y, Martinoia E (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci 107(49):21187–21192
Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, Ma JF (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci 111(44):15699–15704
Soto G, Fox R, Ayub N, Alleva K, Guaimas F, Erijman EJ, Mazzella A, Amodeo G, Muschietti J (2010) TIP5; 1 is an aquaporin specifically targeted to pollen mitochondria and is probably involved in nitrogen remobilization in Arabidopsis thaliana. Plant J 64(6):1038–1047
Srivastava S, Suprasanna P, D’Souza SF (2011) Redox state and energetic equilibrium determine the magnitude of stress in Hydrilla verticillata upon exposure to arsenate. Protoplasma 248(4):805–815
Srivastava S, Pathak S, Ponsin M, Hensawang S, Chanpiwat P, Yoeurn C, Phan K (2021) Sustainable Solutions to arsenic accumulation in rice grown in south and Southeast Asia. Crop Pasture Sci. https://doi.org/10.1071/CP21033
Sun SK, Chen Y, Che J, Konishi N, Tang Z, Miller AJ, Zhao MJF, FJ, (2018) Decreasing arsenic accumulation in rice by overexpressing Os NIP 1; 1 and Os NIP 3; 3 through disrupting arsenite radial transport in roots. New Phytol 219:641–653
Sun D, Feng H, Li X, Ai H, Sun S, Chen Y, Xu G, Rathinasabapathi B, Cao Y, Ma LQ (2019) Expression of new Pteris vittata phosphate transporter PvPht1; 4 reduces arsenic translocation from the roots to shoots in tobacco plants. Environ Sci Technol 54(2):1045–1053
Takanashi K, Shitan N, Yazaki K (2014) The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol 31:417–430
Tanaka Y, Iwaki S, Tsukazaki T (2017) Crystal structure of a plant multidrug and toxic compound extrusion family protein. Structure 25(9):1455–1460
Tang Z, Chen Y, Chen F, Ji Y, Zhao FJ (2017) OsPTR7 (OsNPF8. 1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain. Plant Cell Physiol 58(5):904–913
Tang Z, Chen Y, Miller AJ, Zhao FJ (2019) The C-type ATP-binding cassette transporter OsABCC7 is involved in the root-to-shoot translocation of arsenic in rice. Plant Cell Physiol 60(7):1525–1535
Thakur S, Choudhary S, Dubey P, Bhardwaj P (2019) Comparative transcriptome profiling reveals the reprogramming of gene networks under arsenic stress in Indian mustard. Genome 62:833–847
Thakur S, Choudhary S, Majeed A, Singh A, Bhardwaj P (2020) Insights into the molecular mechanism of arsenic phytoremediation. J Plant Growth Regul 39:532–543
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Sanjib P (2012) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39
Thounaojam TC, Khan Z, Upadhyaya H (2020) Molecular physiology of arsenic uptake, transport, and metabolism in rice. In: Srivastava S (ed) Arsenic in drinking water and food. Springer, Singapore, pp 391–410
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP 1, in arsenic transport and tolerance. Plant cell environ. 37(1):140–152
Upadhyay MK, Majumdar A, Barla A, Bose S, Srivastava S (2021) Thiourea supplementation mediated reduction of grain arsenic in rice (Oryza sativa L) cultivars: a two year field study. J Hazard Mater 407:124368
Vatansever R, Filiz E, Ozyigit II (2016) In silico analysis of Mn transporters (NRAMP1) in various plant species. Mol Biol Rep 43(3):151–163
Wang H, Xu Q, Kong YH, Chen Y, Duan JY, Wu WH, Chen YF (2014) Arabidopsis WRKY45 transcription factor activates PHOSPHATE TRANSPORTER1; 1 expression in response to phosphate starvation. Plant Physiol 164(4):2020–2029
Wang P, Zhang W, Mao C, Xu G, Zhao FJ (2016) The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J Exp Bot 67(21):6051–6059
Wawrzycka D, Markowska K, Maciaszczyk-Dziubinska E, Migocka M (1859) Wysocki R (2017) Transmembrane topology of the arsenite permease Acr3 from Saccharomyces cerevisiae. Biochim Biophys Acta (BBA) Biomembr 1:117–125
Wojas S, Hennig J, Plaza S, Geisler M, Siemianowski O, Skłodowska A, Antosiewicz DM (2009) Ectopic expression of Arabidopsis ABC transporter MRP7 modifies cadmium root-to-shoot transport and accumulation. Environ Pollut 157(10):2781–2789
Wu ZC, Ren HY, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157:498–508
Xiao W, Liu P, Wang K, Yang Z, Wang L (2021) Relationship between ionomics and transcriptomics of rice plant in response to arsenite stress. Environ Exp Bot 24:104565
Xu W, Dai W, Yan H, Li S, Shen H, Chen Y, Xu H, Sun Y, He Z, Ma M (2015) Arabidopsis NIP3; 1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol plant 8(5):722–733
Yadav S, Kushwaha HR, Kumar K, Verma PK (2012) Comparative structural modeling of a monothiol GRX from chickpea: Insight in iron–sulfur cluster assembly. Int J Biol Macro 51(3):266–273
Yadav B, Dubey R, Gnanasekaran P, Narayan OP (2021) OMICS approaches towards understanding plant’s responses to counterattack heavy metal stress: an insight into molecular mechanisms of plant defense. Plant Gene 28:100333
Yan H, Gao Y, Wu L, Wang L, Zhang T, Dai C, Xu W, Feng L, Ma M, Zhu Y-G, He Z (2019) Potential use of the Pteris vittata arsenic hyperaccumulation-regulation network for phytoremediation. J Hazard Mater 368:386–396
Yu LJ, Luo YF, Liao B, Xie LJ, Chen L, Xiao S, Li JT, Hu SN, Shu WS (2012) Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol 195:97–112
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul 37:1331–1348
Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF (2010) The role of the rice aquaporin Lsi1 in arsenite efflux from roots. New Phytol 186(2):392–399
Zvobgo G, LwalabaWaLwalaba J, Sagonda T, Mapodzeke JM, Muhammad N, Shamsi IH, Zhang G (2018) Phosphate alleviates arsenate toxicity by altering expression of phosphate transporters in the tolerant barley genotypes. Ecotoxicol Envirol Saf 147:832–839
Acknowledgements
Thorny Chanu Thounaojam and Hrishikesh Upadhyaya greatly acknowledge the financial support from the Department of Science and Technology (DST), Government of India, under WOS-A scheme (Reference No: SR/WOS-A/LS-159/2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thounaojam, T.C., Khan, Z., Meetei, T.T. et al. Transporters: the molecular drivers of arsenic stress tolerance in plants. J. Plant Biochem. Biotechnol. 30, 730–743 (2021). https://doi.org/10.1007/s13562-021-00748-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-021-00748-z